Market Access Standard Path
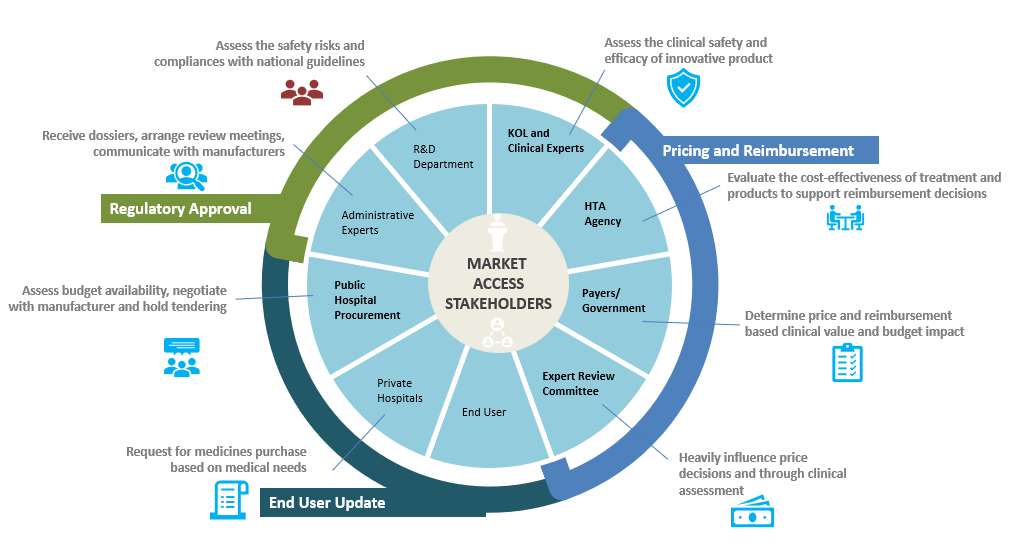
Market access entails a range of essential activities. It begins with a comprehensive Market Access Environment Assessment, encompassing an understanding of diverse healthcare systems, disease management, clinical evidence, and reimbursement dynamics. Value creation involves precise clinical data development and Health Economics & Outcomes Research (HEOR) data, defining endpoints, observed populations, and comparators. Value identification relies on robust Randomized Clinical Trials (RCTs), HEOR data, and insights from payers. Effective value communication involves well-crafted Value Propositions and Dossiers, developed in collaboration with key decision makers. The process is dynamic, involving continuous Real World Evidence data development, potential repositioning, and product re-pricing to ensure ongoing market success


![recruitment20numhom-1[1] recruitment20numhom-1[1]](https://equantx.com/wp-content/uploads/2024/02/recruitment20numhom-11.jpg)