eQuantX help in building an-evidence based value frameworks and pricing strategies to optimize your product’s market access plans around the world. Our team of market access experts support you in accelerating your product approvals, assessing market risk, establishing appropriate pricing, and increasing speed to market for new and innovative products
How we do it
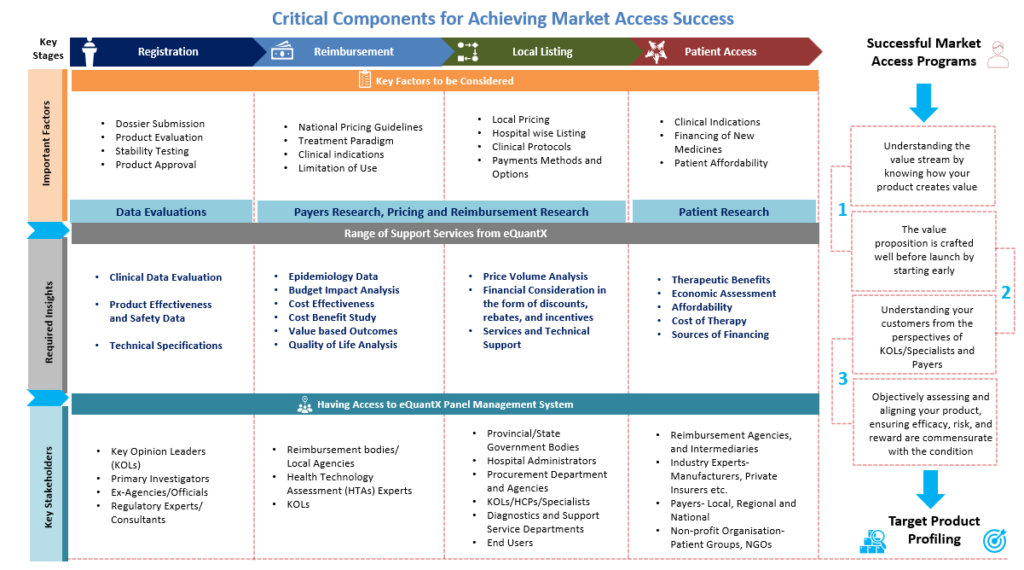
1. Market Access Environment Assessment
This usually performed at the starting of the phase-II studies and focused on the following information to be gathered
 Country Healthcare System:- Understanding country-specific healthcare systems and their requirements
Country Healthcare System:- Understanding country-specific healthcare systems and their requirements Epidemiology Study :- Understanding of disease management and unmet needs
Epidemiology Study :- Understanding of disease management and unmet needs Clinical Evidence Analysis- Understanding of which clinical evidence seems to have mostly impacted in the past
Clinical Evidence Analysis- Understanding of which clinical evidence seems to have mostly impacted in the past Reimbursement Scenario- Country reimbursement status of current treatments and existing restrictions
Reimbursement Scenario- Country reimbursement status of current treatments and existing restrictions


2. Designing Preliminary Market Access Plan
The preliminary market access plan is developed based on initial reaction of payers and physicians on the drafted Target Product Profile (TPP). This step plans specific studies and analysis as follows
 Disease Background
Disease Background Payer Insight Generation
Payer Insight Generation Testing and Adapting Target Product Profile
Testing and Adapting Target Product Profile Pricing Study
Pricing Study Payer Engagement Capability Building
Payer Engagement Capability Building Reimbursement Opportunities and Strategy
Reimbursement Opportunities and Strategy Clinical/economic Impact Analysis/ Budget Impact Analysis
Clinical/economic Impact Analysis/ Budget Impact Analysis
3. Gap Analysis
After discussion on the given TPP, gap analysis is performed to check the evidence require for desired market access positioning and to achieve ultimate commercial goals. This step has clearly defined objectives and stages to create value through significant development of clinical data and HEOR data/ RWE insights generation.
The output from this Gap Analysis is used into phase III clinical and Real-World Evidence research programs.


4.Designing Final Market Access Plan
Through continuous RWE data development plan and analysis, and once the clinical trial results are available from phase III studies, the Market Access plan can be finalized along with the estimated timelines. A subsequent repositioning/ re-pricing strategies could also be proposed and planned accordingly
 Disease Background
Disease Background Developing Negotiation Pathways etc.
Developing Negotiation Pathways etc. Repositioning Strategy
Repositioning Strategy Re-Pricing Strategy
Re-Pricing Strategy
ll FEATURED ACTIVITIES ll
ll FEATURED PRODUCTS ll
What Differentiates Us
Impact by numbers
access support planning for the developing
pipeline assets
research completed in
US and EU markets
Case Studies