Country HTA Snapshots: Germany, France, UK
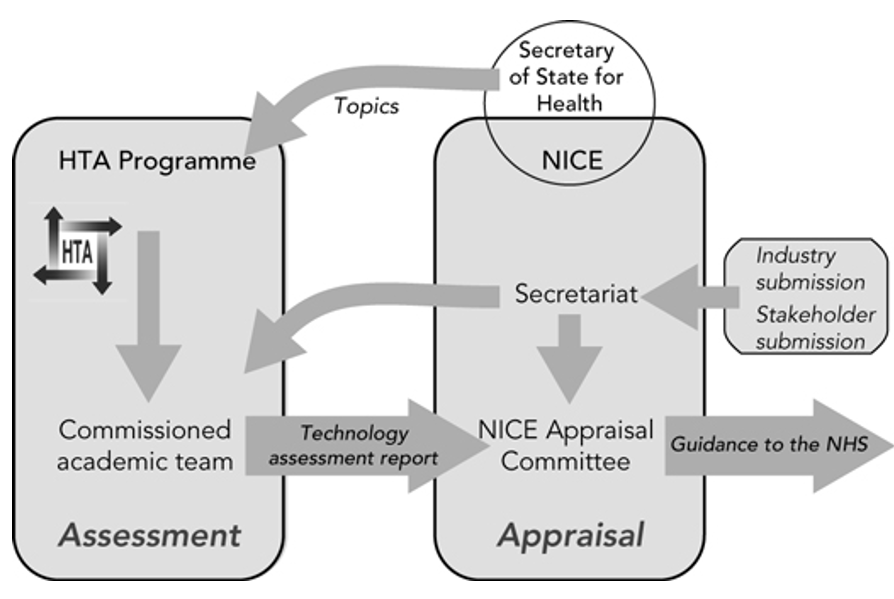
HTA can occur at national or regional levels within a country, with distinct bodies often focusing on different medical interventions. For instance, there are HTA bodies dedicated to evaluating the value of new drugs, while others assess the purpose and value of medical devices or surgical procedures. Notable examples of HTA bodies include the National Institute for Health and Care Excellence (NICE) in England and Wales, the Gemeinsamer Bundesausschuss (GBA) in Germany, often guided by an initial economic evaluation by the Institute for Quality and Efficiency in Healthcare (IQWIG), and Haute Autorité de Santé (HAS) in France.
Understanding the impact of a treatment should be shaped by how patients perceive it, but traditional Health Technology Assessment (HTA) processes often focus on payers, industry, and healthcare perspectives. It’s crucial to integrate the patient voice into value assessments. HTA systems vary in strengths and weaknesses. In Asia, meaningful patient participation is lacking, with Japan having no formal patient role. South Korea and Taiwan show limited patient input with minimal impact. In contrast, states like Australia, Germany, and France demonstrate more inclusive approaches.
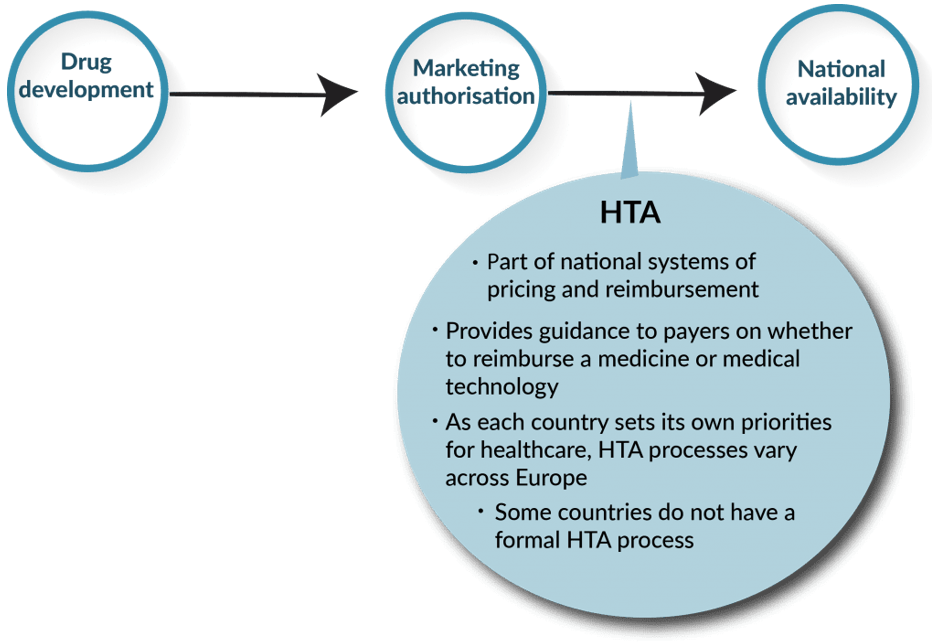
Patients play a role in different stages of the HTA process, but transparency issues pose challenges for patients and their advocates. This challenge manifests in three main ways: firstly, unclear guidance on participating in the process; secondly, structures that may not align with the intended purpose; and thirdly, inadequate communication from HTA bodies regarding the impact of patient input. Even in cases where patients are involved throughout, such as with England’s NICE, rare disease groups may struggle due to their small size, necessitating additional support to navigate the intricacies of the HTA process.
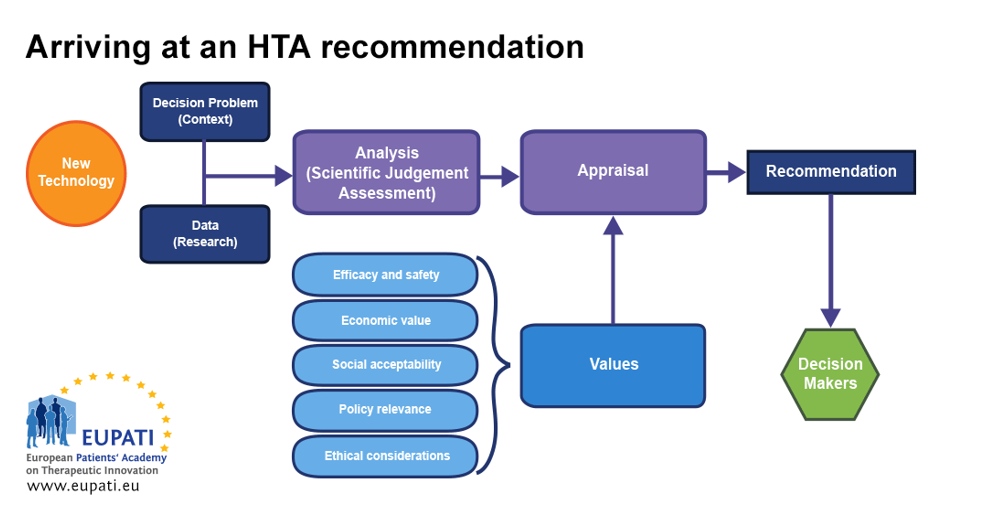
The HTA Network is set to enhance the quality of practices and methods in response to the diverse landscape of European HTA practices and outcomes. By minimizing duplicated efforts, it seeks to optimize the use of HTA resources in Europe. EUnetHTA is actively involved in shaping the HTA Network’s initiatives, including crafting HTA methodology guidelines and piloting joint assessments of relative effectiveness. These efforts aim to streamline the workload at the national level, making it more manageable for Member State HTA bodies to carry out specific analyses and decision-making tailored to their health systems.
In Germany, insurers are obligated to cover the full cost of any new drug upon approval, especially for orphan drugs approved by the European Medicines Agency (EMA). Rapid coverage is ensured, however, drug companies are required to provide real-world data on effectiveness and cost. After a year, a reassessment occurs based on this evidence. The outcome could be confirmation of the price (automatic if the cost stays below €50m), renegotiation, further evidence gathering, or discontinuation of coverage.
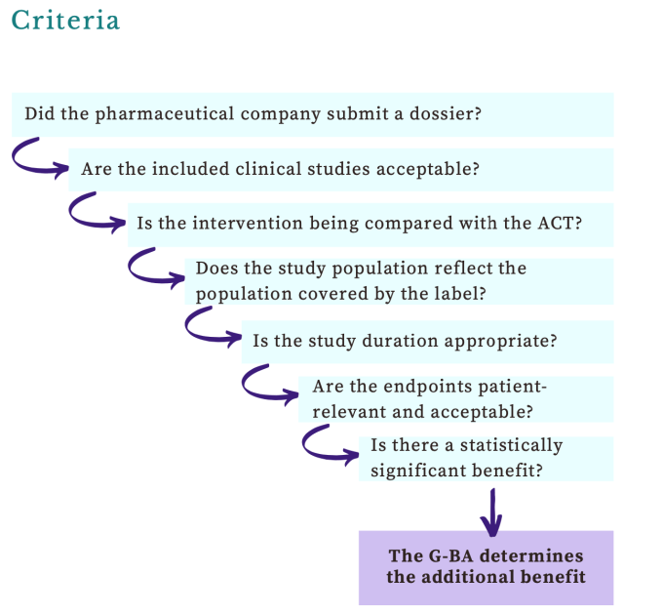
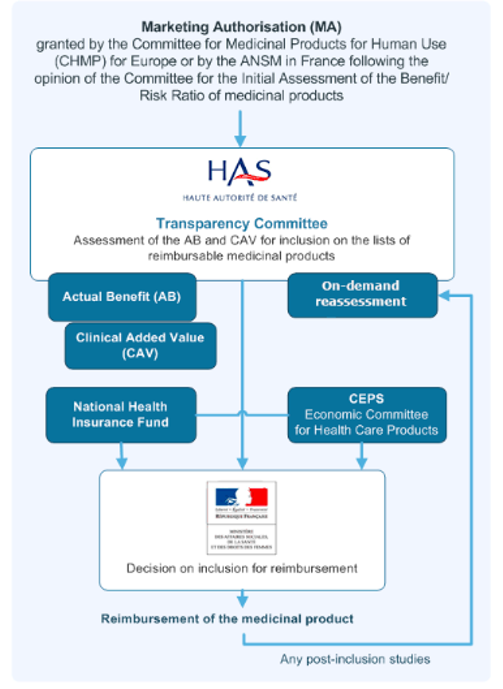
In France, the formal approval process for reimbursement takes more time compared to our examinations of other European countries. After the EMA grants approval for an orphan drug, France’s Haute Autorité de santé (HAS) conducts a comprehensive cost-benefit analysis. Assessments of orphan drugs have the flexibility to consider a broader range of value-related evidence than evaluations of other products.
If the annual cost of the drug to the health system is under €30m, the producer’s price is accepted; otherwise, the HAS outcomes contribute to extended price negotiations between the Comité économique des produits de santé (CEPS), and the drug producer, often leading to prolonged discussions. In the meantime, France has implemented early access programs to facilitate access from the time of or even before EMA approval. However, if consensus is not reached between the producer and CEPS, coverage of the drug may cease.
In UK, the English National Health Service (NHS) approval process is comparable to Germany, and does so with remarkable speed. The expeditious process is attributed to NICE, the HTA body, which shows a readiness to evaluate the case for reimbursement even before a drug secures regulatory approval. Unlike Germany, NICE has a formal willingness-to-pay level, representing an upper limit on price, which is notably higher—five to ten times per quality-adjusted life year (QALY)—for orphan drugs compared to non-orphan ones, contingent on circumstances. In a reform introduced in 2022, NICE affords greater flexibility to assessors of rare disease treatments, allowing them to consider a broader spectrum of evidence in determining the value of a new product.